How to Choose a Ligasure: Essential Buying Factors
Introduction: The Evolution of Hemostatic Control
In contemporary surgical practice, achieving consistent and rapid hemostasis is non-negotiable for optimizing patient outcomes and operative efficiency. Advanced vessel sealing technology, represents a significant leap beyond conventional techniques like sutures and standard electrocautery. These systems deliver a permanent, pressure-resistant seal on vessels and tissue bundles. However, the market offers various devices with differing capabilities. For surgical teams and procurement specialists evaluating this critical investment, a deep understanding of both performance benchmarks and practical operational factors is essential. This guide details the critical elements to assess, ensuring your selected system aligns with clinical demands, workflow integration, and long-term value.

1. Foundational Performance: Efficacy, Safety, and Precision
The cornerstone of any vessel sealing device is its ability to create a reliable seal consistently. Prioritize systems that excel in these core metrics:
- Seal Integrity and Capacity: Seek validation for sealing vessels up to 7mm in diameter. The resulting seal must demonstrate resilience against pressures significantly exceeding systolic blood pressure, a crucial safety factor for all patients, including those with cardiovascular considerations.
- Intelligent Sealing Algorithms: Modern systems should feature adaptive tissue feedback technology. By automatically modulating energy delivery based on real-time tissue impedance, these devices achieve a complete seal—typically signaled by an audible prompt—within a 2 to 6-second window. This intelligence maximizes OR efficiency while safeguarding seal quality.
- Minimized Thermal Effects: A key differentiator is the extent of lateral thermal spread. Leading-edge devices confine thermal damage to ≤2mm from the seal site. This precision, achieved through controlled energy delivery and advanced jaw design, is vital for preserving perioperative tissue integrity, reducing postoperative discomfort, and protecting adjacent anatomical structures.
2. Ergonomic Design and Operational Usability
A device that feels like a natural extension of the surgeon's skill enhances procedural fluidity and safety.
- Generator Simplicity: The ideal generator operates with autonomous energy management, removing the variable of manual power settings. Streamlined features, such as one-button activation on the handpiece and unambiguous visual/auditory sealing confirmation, reduce cognitive load.
- Instrument Versatility: Surgical diversity demands instrument flexibility. A comprehensive portfolio should include specialized handpieces like a 5mm Maryland Jaw for laparoscopic precision dissection, a 5mm Blunt Tip for general laparoscopy, and a 13mm Open Tissue Sealer for open surgeries. This versatility supports cross-specialty adoption.
- Handpiece Engineering: Evaluate ergonomic details: curved jaw designs for enhanced grip and dissection, textured surfaces to prevent tissue slippage, and advanced non-stick coatings (e.g., ceramic). These features minimize tissue adhesion and char buildup, maintaining a clear operative field.
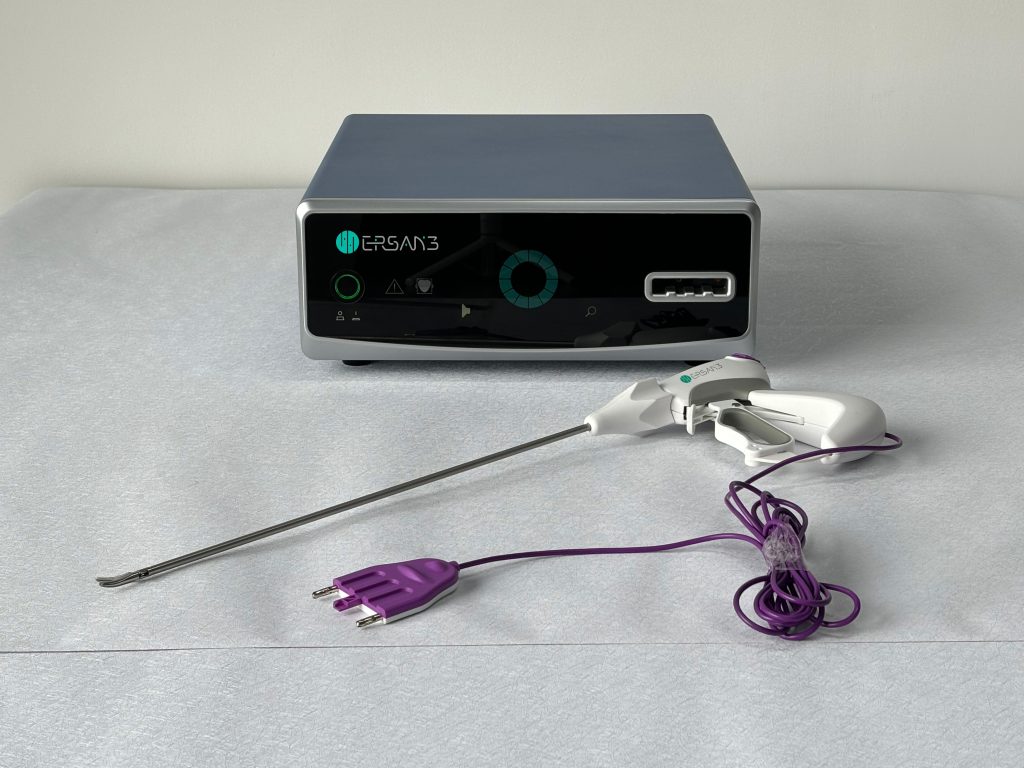
3. System Compatibility and Practical Integration
Seamless integration into existing infrastructure is a major practical determinant, particularly for facilities upgrading or expanding their capabilities.
Platform Interoperability
If your operating suites utilize a specific generator platform (e.g., Medtronic's ForceTriad™ or LS10), verify cross-compatibility with new sealing handpieces. Some scenarios may require adapters. Alternatively, a high-performance, standalone system can offer a unified solution.
Instrument Reprocessing and Lifecycle
Adhere strictly to manufacturer guidelines regarding sterility. While many handpieces are designated single-use, specific models may be safely reprocessed multiple times (e.g., 6-10 cycles) using validated low-temperature sterilization methods (like hydrogen peroxide plasma or ethylene oxide). Clarify the approved reprocessing protocol and expected functional lifespan per instrument to accurately calculate operational costs.
4. Assessing Long-Term Value and Support
The acquisition cost is merely the initial component of the total investment.
- Generator Durability and Warranty: A robust generator is engineered for a long service life, typically 5-8 years or more. A comprehensive warranty (e.g., one year covering parts and labor) provides essential financial and operational protection.
- Cost-Per-Use Economics: Although a consumable, the number of dependable procedures per handpiece directly influences long-term expenditure. Factors such as construction quality, coating durability, and sterilization tolerance define instrument longevity. A higher initial cost for a more durable handpiece often translates to a lower cost-per-procedure over time.
- Technical and Service Support: Access to responsive, expert technical support is critical. Suppliers should provide immediate troubleshooting assistance, clear interpretation of system alerts, and efficient repair services to minimize operational downtime.
5. Evaluating Manufacturer Provenance and Validation
The manufacturer's expertise underpins device reliability, innovation, and compliance.
- Specialized Engineering Heritage: Preference should be given to manufacturers with a dedicated, long-term focus on electrosurgical innovation. A history spanning decades often correlates with refined quality control and clinically-informed design.
- Regulatory and Quality Attestations: Require evidence of international certifications such as CE marking, ISO 13485 quality management systems, and FDA clearance/approval for relevant markets. These attest to adherence to rigorous safety and performance standards.
- Global Clinical Adoption: A manufacturer with a proven export history across diverse international markets (e.g., North America, Europe, Asia-Pacific) has demonstrated the ability to meet varied regulatory and clinical requirements, indicating product robustness and reliability.
Conclusion: A Strategic Decision for Surgical Excellence
Selecting an advanced vessel sealing system is a strategic choice with direct implications for patient care, operational efficiency, and fiscal management. It necessitates a balanced evaluation of uncompromising performance, seamless usability, sustainable cost-of-ownership, and unwavering support.
Why Partner with 233 Medical for Your Vessel Sealing Needs?
For over 16 years, 233 Medical has specialized in the engineering and manufacture of advanced electrosurgical solutions, including our high-fidelity vessel sealing systems. Our design philosophy directly addresses the critical criteria outlined above. We provide intelligent generator platforms (250W-300W) that ensure rapid, reliable seals with minimal thermal spread, coupled with a comprehensive suite of ergonomic handpieces featuring durable, non-stick coatings for superior performance.
Operating as a factory-direct source allows us to offer exceptional value while maintaining the stringent quality standards demanded in operating rooms worldwide. Our systems, trusted by professionals in over 100 countries, are supported by full international certifications, a robust 1-year warranty, and dedicated technical support. We are committed to partnering with your facility to enhance surgical outcomes.
To explore how a 233 Medical vessel sealing system can meet the specific needs of your surgical practice, we invite you to contact our team for a detailed consultation, product specifications, and discussion of procurement options.